A Breast Cancer Vaccine Could Soon Be Available
We usually think of vaccines as safeguards against infectious diseases, like the flu and Covid – not as a cure for cancer. In fact, some vaccines can effectively prevent cancer, but they do so by targeting cancer-causing viruses, like the human papillomavirus (a common cause of cervical cancer). When it comes to most cancers, though, scientists have struggled to create a vaccine that helps the body’s immune system destroy cancer cells. But all of that is about to change: Cleveland Clinic researchers have developed a vaccine against a specific type of extremely aggressive breast cancer.
The vaccine would target triple-negative breast cancer, a cancer type that tests negative for estrogen receptors, progesterone receptors, and excess HER2 protein. In other words, neither these hormones nor the HER2 protein fuel triple-negative cancer, so finding a successful treatment is difficult. And while only 12 to 15 percent of all breast cancers are triple negative, this aggressive form of the disease accounts for a much higher percentage of deaths than other breast cancers, and is more likely to recur — so this new study could help save a lot of lives.
How does the breast cancer vaccine work?
According to the primary vaccine creator, Dr. Vincent Tuohy, Ph.D., and the lead investigator of the study, G. Thomas Budd, M.D., the vaccine targets a specific protein, α-lactalbumin, that emerges in most women who are diagnosed with triple-negative breast cancer, by stimulating the immune system to attack and destroy cells that express the α-lactalbumin protein before they can grow into tumors. In addition, the vaccine activates an innate immune response that halts the growth of emerging tumors.
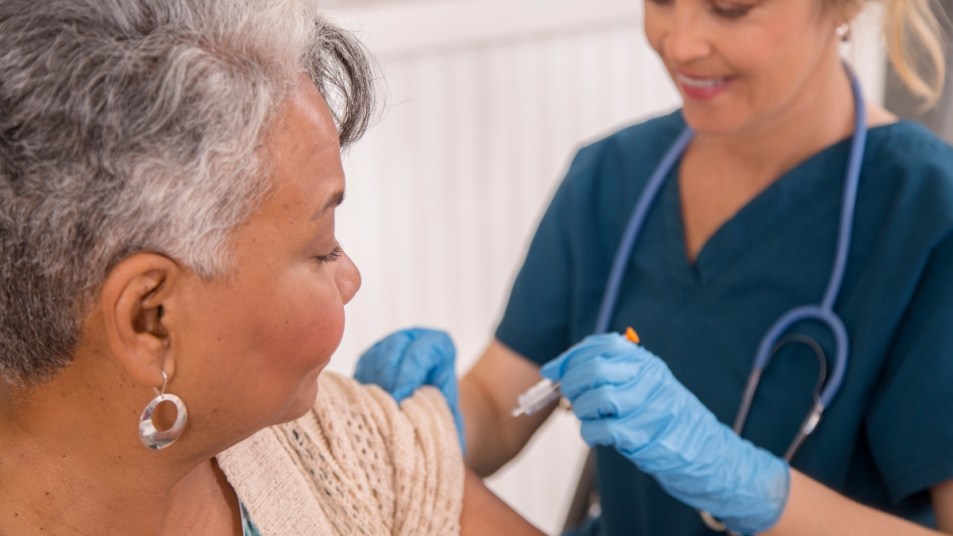
In the pre-clinical research published in Nature Medicine, Dr. Tuohy and his team found that just one dose of the vaccine prevented triple-negative breast tumors from growing in mice. That impressive data convinced the Food and Drug Administration (FDA) to approve a human study, which is being funded by the U.S. Department of Defense.
What needs to happen before the vaccine is approved?
Dr. Budd, lead researcher and staff physician at the Cleveland Clinic Taussig Cancer Center, told Time that the first phase will test the safety of the vaccine. It will include 18 to 24 women who have triple-negative breast cancer and were previously treated with traditional therapies. (Those include chemotherapy, surgery, and DNA-interfering drugs that prevent cancer cells from multiplying.)
Each participant will receive three doses of the vaccine spaced out over six weeks. After that, they’ll be evaluated for side effects. Researchers will also test whether the vaccine activates their immune systems against cancer cells. Dr. Budd notes that later on, they will monitor the women to see whether the cancer returns.
If the vaccine is found to be safe and effective, they will expand the study to include women who don’t yet have the disease, but have a high risk of developing it. Proving that the vaccine works as a preventive method against triple-negative breast cancer could be revolutionary. Better yet, the team believes that their research could help create new vaccine strategies for other types of tumors.
When combined with recent advancements in breast cancer detection, a longer life expectancy for people who have, or have had, breast cancer may be just around the corner.